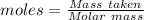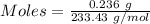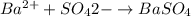Chemistry, 30.10.2019 03:31 putaprincess16
Asolution contains an unknown mass of dissolved barium ions. when sodium sulfate is added to the solution, a white precipitate forms. the precipitate is filtered and dried and then found to have a mass of 236 mg. what mass of barium was in the original solution? (assume that all of the barium was precipitated out of solution by the reaction.)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Summarize possible ways in which phases of matter could combine to form a solution.
Answers: 2

Chemistry, 22.06.2019 13:10
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1

Chemistry, 22.06.2019 23:00
What is the oxidation state of an individual bromine atom in nabro3?
Answers: 2

You know the right answer?
Asolution contains an unknown mass of dissolved barium ions. when sodium sulfate is added to the sol...
Questions



History, 04.01.2020 04:31

Social Studies, 04.01.2020 04:31

Social Studies, 04.01.2020 04:31


Biology, 04.01.2020 04:31




Mathematics, 04.01.2020 04:31



Mathematics, 04.01.2020 04:31

Mathematics, 04.01.2020 04:31



English, 04.01.2020 04:31


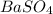 obtained on precipitation = 236 mg
obtained on precipitation = 236 mg