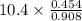Chemistry, 18.10.2019 19:10 Chrissyx4750
Suppose the reaction between nitrogen and hydrogen was run according to the amounts presented in part a, and the temperature and volume were constant at values of 303 k and 2.00 l, respectively. if the pressure was 10.4 atm prior to the reaction, what would be the expected pressure after the reaction was completed?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 13:00
Jose and eric were given four samples in lab. the results of their analysis are shown in the table. based on the data they collected, which sample is most likely a metal?
Answers: 1

Chemistry, 22.06.2019 15:20
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1

Chemistry, 22.06.2019 21:00
One similarity and one difference between an element and a mixture of elements
Answers: 1

Chemistry, 23.06.2019 06:30
Consider the heating curve of h2o and line segments a, b, and c. several changes are taking place at a, b, and c. all but one would be an appropriate description as e move through segments a, b and then c.
Answers: 3
You know the right answer?
Suppose the reaction between nitrogen and hydrogen was run according to the amounts presented in par...
Questions

Biology, 02.01.2021 21:20



Mathematics, 02.01.2021 21:20


Mathematics, 02.01.2021 21:30

Mathematics, 02.01.2021 21:30


Mathematics, 02.01.2021 21:30


Mathematics, 02.01.2021 21:30

Mathematics, 02.01.2021 21:30



Mathematics, 02.01.2021 21:30

Mathematics, 02.01.2021 21:30


Mathematics, 02.01.2021 21:30


Biology, 02.01.2021 21:30

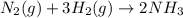
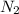 + moles of
+ moles of 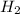 = 0.908 mol
= 0.908 mol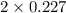 = 0.454 mol
= 0.454 mol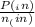 =
= 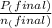
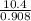 =
= 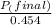
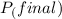 =
=