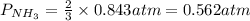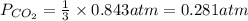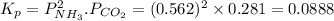Chemistry, 18.10.2019 19:10 daverius3153
7. nh2co2nh4(s) when heated to 450 k undergoes the following reaction to produce a system which reaches equilibrium: nh2co2nh4(s) ⇀↽ 2 nh3(g) + co2(g) the total pressure in the closed container under these condition is found to be 0.843 atm. calculate a value for the equilibrium constant, kp. a) 0.00701 b) 0.0888 c) 0.222 d) 0.599

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:10
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1

Chemistry, 22.06.2019 13:30
Astudent is trying to create a table that compares hypotheses, theories, and laws. hypothesis theory law do scientific researchers formulate it? yes yes yes does it explain why things happen? yes yes no yes yes yes is it used to make predictions? no yes yes which of the following questions would most likely fill the blank in the table? is it an intelligent guess? is it newly formulated? is it based on observations? has it been proved?
Answers: 1

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical systems? a) water dissolves nonpolar ionic compounds. b) water dissociates ionic compounds. c) water dissociates covalent molecules. d) water dissolves nonpolar covalent substances.
Answers: 1

Chemistry, 22.06.2019 20:00
What happens to the temperature of a substance when the average kinetic energy of its particles increases?
Answers: 3
You know the right answer?
7. nh2co2nh4(s) when heated to 450 k undergoes the following reaction to produce a system which reac...
Questions


Computers and Technology, 21.06.2021 20:00

Mathematics, 21.06.2021 20:00



Mathematics, 21.06.2021 20:00

Mathematics, 21.06.2021 20:00





Mathematics, 21.06.2021 20:00

Mathematics, 21.06.2021 20:00


Social Studies, 21.06.2021 20:00



Mathematics, 21.06.2021 20:00

Mathematics, 21.06.2021 20:00

English, 21.06.2021 20:00

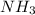 and
and 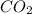 are gaseous. Hence equilibrium constant depends upon partial pressures of
are gaseous. Hence equilibrium constant depends upon partial pressures of 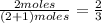
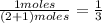
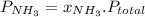 and P_{CO_{2}}= x_{CO_{2}}.P_{total}
and P_{CO_{2}}= x_{CO_{2}}.P_{total}