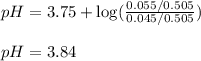Chemistry, 28.09.2019 02:20 mikailah0988
A500 ml sample of a 0.100 m formate buffer, ph 3.75, is treated with 5 ml of 1.00 m koh. what is the ph following this addition?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:40
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2

Chemistry, 22.06.2019 09:30
Which element is the least metallic between cadmium, silver, zinc, or iron?
Answers: 1

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1

Chemistry, 22.06.2019 15:00
Large helium-filled balloons are used to lift scientific equipment to high altitudes. what is the pressure inside such a balloon if it starts out at sea level with a temperature of 10.0ºc and rises to an altitude where its volume is twenty times the original volume and its temperature is – 50.0ºc ?
Answers: 2
You know the right answer?
A500 ml sample of a 0.100 m formate buffer, ph 3.75, is treated with 5 ml of 1.00 m koh. what is the...
Questions

Mathematics, 16.12.2020 18:10

Biology, 16.12.2020 18:10


English, 16.12.2020 18:10

Spanish, 16.12.2020 18:10




Mathematics, 16.12.2020 18:10

Mathematics, 16.12.2020 18:10


Biology, 16.12.2020 18:10

Mathematics, 16.12.2020 18:10

Chemistry, 16.12.2020 18:10

Mathematics, 16.12.2020 18:10




Mathematics, 16.12.2020 18:10

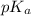 of formic acid = 3.75
of formic acid = 3.75![pH=pK_a+\log(\frac{[HCOO-]}{[HCOOH]})](/tpl/images/0269/8215/7d130.png)
![3.75=3.75+\log(\frac{[HCOO-]}{[HCOOH]})\\\\\log(\frac{[HCOO-]}{[HCOOH]})=0\\\\\frac{[HCOO-]}{[HCOOH]}=1](/tpl/images/0269/8215/85595.png)
![[HCOO-]=[HCOOH]](/tpl/images/0269/8215/5cab3.png)
![[HCOO-]+[HCOOH]=0.1](/tpl/images/0269/8215/4bfcc.png)
![[HCOO-]=[HCOOH]=0.05M](/tpl/images/0269/8215/44a66.png)
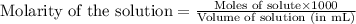
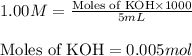
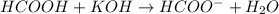
![pH=pK_a+\log(\frac{[salt]}{[acid]})](/tpl/images/0269/8215/e4eea.png)
![pH=pK_a+\log(\frac{[HCOO^-]}{[HCOOH]})](/tpl/images/0269/8215/e9614.png)
![[HCOO^-]=\frac{0.055}{0.505}](/tpl/images/0269/8215/914e5.png)
![[HCOOH]=\frac{0.045}{0.505}](/tpl/images/0269/8215/1cfaa.png)