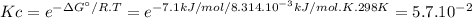Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Explain why scientists use shared characteristics to make cladograms.
Answers: 1

Chemistry, 22.06.2019 02:30
Asa choose the correct set of reaction coefficients to properly balance the following chemical equation according to the law of conservation of mass: __s8 + __o2 ==> __so2 1, 1, 8 1, 8, 1 1, 8, 8 8, 1, 1
Answers: 1

Chemistry, 22.06.2019 08:30
Which common material is an example of a polymer? (25 pts) a. steel b. plastic c. petroleum d. rubbing alcohol
Answers: 2

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 3
You know the right answer?
Ag' for the isomerization reaction glucose-1-phosphate (gip) $ glucose-6-phosphate (g6p) is -7.1 kj/...
Questions






Mathematics, 25.06.2019 16:00

Computers and Technology, 25.06.2019 16:00

Spanish, 25.06.2019 16:00


Mathematics, 25.06.2019 16:00

Spanish, 25.06.2019 16:00


Mathematics, 25.06.2019 16:00


Biology, 25.06.2019 16:00

History, 25.06.2019 16:00


Mathematics, 25.06.2019 16:00


Mathematics, 25.06.2019 16:00

![Kc=\frac{[G1P]}{[G6P]}](/tpl/images/0269/8214/83bf5.png)