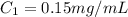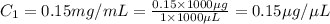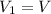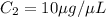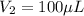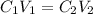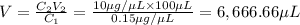You are given a protein solution with a concentration of 0.15 mg/ml.
v. suppose that we...

Chemistry, 28.09.2019 02:20 Pandorasbx2657
You are given a protein solution with a concentration of 0.15 mg/ml.
v. suppose that we want to prepare 100 microliters of 10 micrograms/microliters solution. how much of h2o and protein stock do we need to add to obtain the target concentration and volume? (the concentration is not greater in the question, you need to convert it to micrograms/ then it should make sense)

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 13:10
What type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? view available hint(s) what type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? salt bridge disulfide bridge hydrogen bond hydrophobic interaction
Answers: 1

Chemistry, 23.06.2019 00:30
There are approximately 15 milliliters (ml) in 1 tablespoon (tbsp). what expression can be used to find the approximate number of milliliters in 3 tbsp?
Answers: 1

You know the right answer?
Questions


Mathematics, 16.02.2021 14:00

Chemistry, 16.02.2021 14:00

Advanced Placement (AP), 16.02.2021 14:00

Mathematics, 16.02.2021 14:00


Chemistry, 16.02.2021 14:00



Mathematics, 16.02.2021 14:00

English, 16.02.2021 14:00


Mathematics, 16.02.2021 14:00

Arts, 16.02.2021 14:00

Social Studies, 16.02.2021 14:00

English, 16.02.2021 14:00


Mathematics, 16.02.2021 14:00

History, 16.02.2021 14:00

English, 16.02.2021 14:00