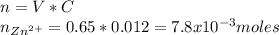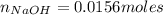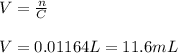Chemistry, 13.09.2019 04:20 ashtor1943
If you had a 0.650 l solution containing 0.0120 m of zn2+(aq), and you wished to add enough 1.34 m naoh(aq) to precipitate all of the metal, what is the minimum amount of the naoh(aq) solution you would need to add? assume that the naoh(aq) solution is the only source of oh−(aq) for the precipitation.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Long term exposure to waves can cause sunburns and skin cancer. a) visible b) infrared c) gamma rays d) ultraviole
Answers: 1

Chemistry, 22.06.2019 09:00
Given the following reaction: c3h8+5o2=3co2+4h20 how many grams of co2 will be produced 7 g of c3h8 and 98 g of o2
Answers: 1

Chemistry, 22.06.2019 23:30
If it is an isoelectronic series select true, if not select false. o2-, s2-, se2-, te2- na+, k+, rb+, cs+ n3-, p3-, as3-, sb3- ag, cd+, sn3+, sb4+ f-, cl-, br-, i- f-, ne, na+, mg2+ s2-, s, s6+
Answers: 1

Chemistry, 23.06.2019 00:10
Covalent compounds: mastery test select the correct answer what is formed when atoms join together with a covalent bond? a. an ion b. a molecule c. a neutral atom d. a noble gas
Answers: 3
You know the right answer?
If you had a 0.650 l solution containing 0.0120 m of zn2+(aq), and you wished to add enough 1.34 m n...
Questions

Mathematics, 12.02.2020 20:24


Mathematics, 12.02.2020 20:24




History, 12.02.2020 20:26



Mathematics, 12.02.2020 20:29

Social Studies, 12.02.2020 20:30






Mathematics, 12.02.2020 20:30

Chemistry, 12.02.2020 20:30