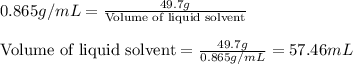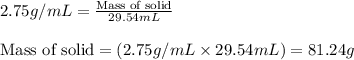A liquid solvent is added to a flask containing an insoluble solid. The total volume of the solid and liquid together is 87.0 mL. The liquid solvent has a mass of 49.7 g and a density of 0.865 g/mL. Determine the mass of the solid given its density is 2.75 g/mL.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Liv sheldon given the balanced equation for an organic reaction: c2h2 + 2cl2 → c2h2cl4 this reaction is best classified as *
Answers: 1

Chemistry, 22.06.2019 08:40
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3

Chemistry, 22.06.2019 13:30
Which is true of a liquid? it has a definite volume but not a definite mass.it has a definite mass but not a definite volume.it has a definite volume but not a definite shape.it has a definite shape but not a definite volume.
Answers: 2

Chemistry, 23.06.2019 00:30
What is the percent by mass of magnesium sulfate in mgso4.7h2o
Answers: 3
You know the right answer?
A liquid solvent is added to a flask containing an insoluble solid. The total volume of the solid an...
Questions





Mathematics, 16.01.2020 22:31

Mathematics, 16.01.2020 22:31

Business, 16.01.2020 22:31

Mathematics, 16.01.2020 22:31






Health, 16.01.2020 22:31

Social Studies, 16.01.2020 22:31

Mathematics, 16.01.2020 22:31

History, 16.01.2020 22:31



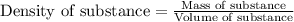 ......(1)
......(1)