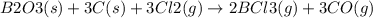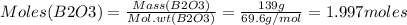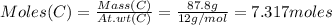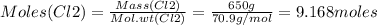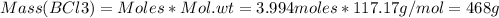Consider the reaction of diboron trioxide with carbon and chlorine. b2o3 (s) + 3c (s) + 3cl2 (g) 2bcl3 (g) + 3co (g) determine the limiting reactant in a mixture containing 139 g of b2o3, 87.8 g of c, and 650 g of cl2. calculate the maximum mass (in grams) of boron trichloride, bcl3, that can be produced in the reaction. the limiting reactant is: b2o3 cl2 c amount of bcl3 formed = g

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 16:50
What is conserved in the reaction shown below? h2(g) + cl2 (g) --> 2hcl(g)a. mass onlyb. mass and moles onlyc. mass, moles, and molecules onlyd. mass, moles, molecules, and volume
Answers: 2

Chemistry, 23.06.2019 03:00
In november 1987, a massive iceberg broke loose from the antartic ice mass and floated free in the ocean. the chunk of ice was estimated to be 98 mi long, 25 mi wide, and 750 ft thick. a typical backyard swimming pool contains about 24,000 gallons of water. how many of these pools could you fill from the water in this iceberg? (assume the iceberg is a rectangular solid of the above dimensions and consists of water only). express answer in scientific notation.
Answers: 3

Chemistry, 23.06.2019 06:30
1.17 mol hcl and 2.5 mol naoh react according to the equation hcl + naoh -> nacl + h2o . if the limiting reactant is hcl, determine the amount of excess reactant that remains. answer in units of mol.
Answers: 1

Chemistry, 23.06.2019 15:00
What is the volume in liters of 7500 g of helium atoms. assume stp conditions.
Answers: 1
You know the right answer?
Consider the reaction of diboron trioxide with carbon and chlorine. b2o3 (s) + 3c (s) + 3cl2 (g) 2bc...
Questions


English, 29.04.2021 01:00

Mathematics, 29.04.2021 01:00




Mathematics, 29.04.2021 01:00


Mathematics, 29.04.2021 01:00


Mathematics, 29.04.2021 01:00

Biology, 29.04.2021 01:00

History, 29.04.2021 01:00


Mathematics, 29.04.2021 01:00

Mathematics, 29.04.2021 01:00




Mathematics, 29.04.2021 01:00