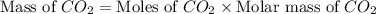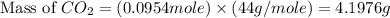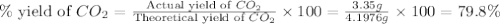Chemistry, 08.08.2019 06:20 redhot12352
Consider the reaction of c3h8 with o2 to form co2 and h2o. if 5.11 g o2 is reacted with excess c3h8 and 3.35 g of co2 is ultimately isolated, what is the percent yield for the reaction? percent yield = %

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
What effect might melting sea ice have for people who live in coastal areas?
Answers: 1

Chemistry, 22.06.2019 11:00
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1

Chemistry, 22.06.2019 14:30
Ahypothesis must be testable and falsifiable to be considered scientific a. trueb. false
Answers: 1

Chemistry, 22.06.2019 14:30
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2
You know the right answer?
Consider the reaction of c3h8 with o2 to form co2 and h2o. if 5.11 g o2 is reacted with excess c3h8...
Questions



Mathematics, 03.11.2021 01:00

English, 03.11.2021 01:00



Mathematics, 03.11.2021 01:00



Mathematics, 03.11.2021 01:00

Mathematics, 03.11.2021 01:00




Mathematics, 03.11.2021 01:00

Computers and Technology, 03.11.2021 01:00

Physics, 03.11.2021 01:00



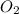 = 5.11 g
= 5.11 g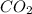 = 44 g/mole
= 44 g/mole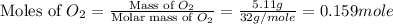
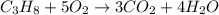
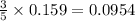 moles of
moles of