
A2.832 gram sample of an organic compound containing c, h and o is analyzed by combustion analysis and 6.439 grams of co2 and 2.636 grams of h2o are produced. in a separate experiment, the molar mass is found to be 116.2 g/mol. determine the empirical formula and the molecular formula of the organic compound. enter the elements in the order c, h, o empirical formula = molecular formula =

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:50
Which of the following electromagnetic waves can create ions?
Answers: 2

Chemistry, 22.06.2019 09:00
What term is missing from the central region that describes hypotheses, theories, and laws? popular predictable mathematical falsifiable
Answers: 2

Chemistry, 22.06.2019 14:30
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3

Chemistry, 22.06.2019 19:30
Acetylene gas c2h2 undergoes combustion to produce carbon dioxide and water vapor how many grams of water are produced by the same amount of c2h2?
Answers: 2
You know the right answer?
A2.832 gram sample of an organic compound containing c, h and o is analyzed by combustion analysis a...
Questions

Mathematics, 07.05.2021 18:20




Mathematics, 07.05.2021 18:20

Mathematics, 07.05.2021 18:20


Mathematics, 07.05.2021 18:20

Mathematics, 07.05.2021 18:20

Mathematics, 07.05.2021 18:20




Mathematics, 07.05.2021 18:20


Mathematics, 07.05.2021 18:20


Mathematics, 07.05.2021 18:20

Mathematics, 07.05.2021 18:20

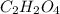 and
and 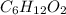 respectively.
respectively.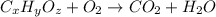
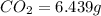

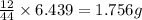 of carbon will be contained.
of carbon will be contained.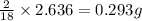 of hydrogen will be contained.
of hydrogen will be contained.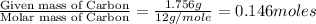
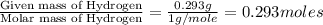
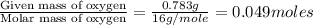
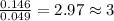
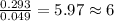
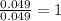
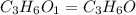
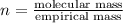
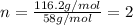
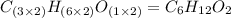
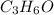 and
and 

