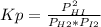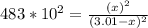Chemistry, 08.08.2019 06:20 sullivanjakob
At a particular temperature, kp-483 × 102 for the reaction h2 (g) + 12(g) ? 2h1(g) if 3 01 atm of h-(g) and 3.01 atm of i2(g) are introduced into a 1.00-l- container, calculate the equilibrium partial pressures of all partial pressure of h2 partial pressure of i2atm partial pressure ofhiatm atm

Answers: 1


Another question on Chemistry




You know the right answer?
At a particular temperature, kp-483 × 102 for the reaction h2 (g) + 12(g) ? 2h1(g) if 3 01 atm of h...
Questions

English, 22.05.2021 03:50




Mathematics, 22.05.2021 03:50


English, 22.05.2021 03:50

Mathematics, 22.05.2021 03:50

Computers and Technology, 22.05.2021 03:50

Mathematics, 22.05.2021 03:50

Mathematics, 22.05.2021 03:50

Mathematics, 22.05.2021 03:50


Mathematics, 22.05.2021 03:50


Biology, 22.05.2021 03:50



Mathematics, 22.05.2021 03:50

Mathematics, 22.05.2021 03:50