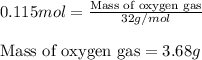Chemistry, 08.08.2019 06:20 lilyrockstarmag
Identify the limiting reactant in the reaction of carbon monoxide and oxygen to form co2, if 9.16 g of co and 9.01 g of o2 are combined. determine the amount (in grams) of excess reactant that remains after the reaction is complete.
formula of limiting reactant =
amount of excess reactant remaining = g

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
This question is about electrolysis. metal spoons can be coated with silver. this is called electroplating. suggest one reason why spoons are electroplated?
Answers: 1


Chemistry, 22.06.2019 19:50
If a gas has an initial pressure of 101kpa and a volume of 10l, then it expands to a volume of 20l, what is the new pressure?
Answers: 2

Chemistry, 23.06.2019 10:40
Aliquid solution can be made select all that apply. dissolving solids into liquids, mixing liquids, dissolving gas solutes into liquids , mixing gases, mixing solids
Answers: 3
You know the right answer?
Identify the limiting reactant in the reaction of carbon monoxide and oxygen to form co2, if 9.16 g...
Questions


Social Studies, 14.09.2021 14:20


Mathematics, 14.09.2021 14:20

Mathematics, 14.09.2021 14:20



Mathematics, 14.09.2021 14:20


History, 14.09.2021 14:20


Mathematics, 14.09.2021 14:20

Spanish, 14.09.2021 14:20

Mathematics, 14.09.2021 14:20


Mathematics, 14.09.2021 14:30

Mathematics, 14.09.2021 14:30



Health, 14.09.2021 14:30

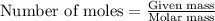 ....(1)
....(1) 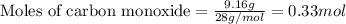
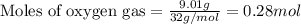
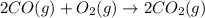
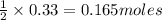 of oxygen gas
of oxygen gas