
Chemistry, 08.08.2019 06:20 xcncxgnfxg6487
According to the following equation, how many ml of 0.15 m naoh would be needed to titrate 10.00 ml of 0.500 m hcl? 16. what is the ph of a 0.33 m solution of hci?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:30
One mole of zinc has a mass of 65.4 grams. approximately how many atoms of zinc are present in one mole of zinc? 32 × 1023 atoms 6 × 1023 atoms 66 atoms 65 atoms
Answers: 1

Chemistry, 21.06.2019 23:00
An electrons position cannot be known precisely only it's probability of being in a certain location can be known
Answers: 1

Chemistry, 22.06.2019 06:00
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1

Chemistry, 22.06.2019 10:00
According to the tide table below what time of day will the highest tide occur? (2 pt) the highest tide will occur at
Answers: 1
You know the right answer?
According to the following equation, how many ml of 0.15 m naoh would be needed to titrate 10.00 ml...
Questions


Computers and Technology, 12.04.2021 17:50

Mathematics, 12.04.2021 17:50



History, 12.04.2021 17:50

Mathematics, 12.04.2021 17:50

Biology, 12.04.2021 17:50

History, 12.04.2021 17:50


Arts, 12.04.2021 17:50

Mathematics, 12.04.2021 17:50


History, 12.04.2021 17:50

English, 12.04.2021 17:50


Mathematics, 12.04.2021 17:50


Mathematics, 12.04.2021 17:50

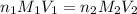
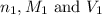 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 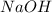
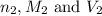 are the n-factor, molarity and volume of base which is HCl.
are the n-factor, molarity and volume of base which is HCl.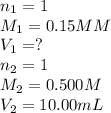
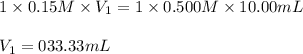
![pH=-\log[H^+]](/tpl/images/0173/1734/cf945.png)
![pH=-\log[0.33 M]=0.48](/tpl/images/0173/1734/35b3a.png)


