Chemistry, 31.07.2019 17:20 drealtania21
Enter your answer in the provided box. sodium hydroxide is used extensively in acid-base titrations because it is a strong, inexpensive base. a sodium hydroxide solution was standardized by titrating 28.58 ml of 0.1851 m standard hydrochloric acid. the initial buret reading of the sodium hydroxide was 1.98 ml, and the final reading was 30.89 ml. what was the molarity of the base solution?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 19:30
Describe the forces both attractive and repulsive that occur as two atoms move closer together.
Answers: 1

Chemistry, 22.06.2019 21:30
In one or two grammatically correct sentences, write a definition for the term molecule geometry
Answers: 3

Chemistry, 22.06.2019 23:30
The density of the solid phase of a substance is 0.90 g/cm3 and the density of the liquid phase is 1.0 g/cm3. a large increase in pressure will a. lower the freezing point b. raise the freezing point c. lower the boiling point d. raise the triple point e. lower the triple point
Answers: 1

Chemistry, 23.06.2019 00:30
Five different substances are given to you to be dissolved in water. which substances are most likely to undergo dissolution in water? check all that apply. view available hint(s) check all that apply. sodium fluoride, naf octane, c8h18 propanol, ch3ch2ch2oh potassium iodide, ki benzene, c6h6
Answers: 1
You know the right answer?
Enter your answer in the provided box. sodium hydroxide is used extensively in acid-base titrations...
Questions

English, 18.12.2020 17:40

English, 18.12.2020 17:40




Business, 18.12.2020 17:40




History, 18.12.2020 17:40

Mathematics, 18.12.2020 17:40

History, 18.12.2020 17:40


Business, 18.12.2020 17:40





Mathematics, 18.12.2020 17:40


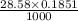 moles
moles