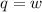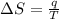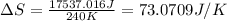Suppose 4.00 mol of an ideal gas undergoes a reversible isothermal expansion from volume v1 to volume v2 = 9v1 at temperature t = 240 k. find (a) the work done by the gas and (b) the entropy change of the gas. (c) if the expansion is reversible and adiabatic instead of isothermal, what is the entropy change of the gas?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Why are the trends and exceptions to the trends in ionization energy observed?
Answers: 1


Chemistry, 22.06.2019 22:30
Akno3 solution containing 51 g of kno3 per 100.0 g of water is cooled from 40 ∘c to 0 ∘c. what will happen during cooling?
Answers: 3

Chemistry, 23.06.2019 14:00
Which of the following is not a result when a change to an equilibrium system is applied? (2 points) increasing the rate of the forward reaction will cause a shift to the left. increasing the rate of the reverse reaction will cause a shift to the left. decreasing the rate of the forward reaction will cause a shift to the left. decreasing the rate of the reverse reaction will cause a shift to the right.
Answers: 1
You know the right answer?
Suppose 4.00 mol of an ideal gas undergoes a reversible isothermal expansion from volume v1 to volum...
Questions

Mathematics, 10.09.2019 21:10



Business, 10.09.2019 21:10


Mathematics, 10.09.2019 21:10


Social Studies, 10.09.2019 21:10












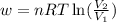
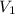 = initial volume of gas =
= initial volume of gas = 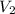 = final volume of gas =
= final volume of gas = 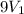
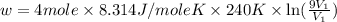
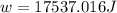
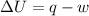
 = internal energy
= internal energy