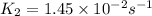Chemistry, 29.07.2019 19:20 faithtunison
The reaction c4h8(g)⟶2c2h4(g) c4h8(g)⟶2c2h4(g) has an activation energy of 262 kj/mol.262 kj/mol. at 600.0 k,600.0 k, the rate constant, , is 6.1×10−8 s−1.6.1×10−8 s−1. what is the value of the rate constant at 785.0 k?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:10
Answer from each drop-down menu. e characteristics of a borane molecule (bh). the lewis structure and table of electronegativities are given olecular shape is and the molecule is reset next erved. search e a
Answers: 2

Chemistry, 22.06.2019 05:20
Temperature is _related to the average kinetic energy of a gas. inversely directly not disproportionally
Answers: 1


Chemistry, 23.06.2019 03:00
In which of the following phases of matter do molecules have the highest amount of energy? a. liquid b. gel c. solid d. gas
Answers: 2
You know the right answer?
The reaction c4h8(g)⟶2c2h4(g) c4h8(g)⟶2c2h4(g) has an activation energy of 262 kj/mol.262 kj/mol. at...
Questions




















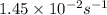
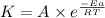
![\log (\frac{K_2}{K_1})=\frac{Ea}{2.303\times R}[\frac{1}{T_1}-\frac{1}{T_2}]](/tpl/images/0147/7591/6d953.png)
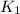 = rate constant at
= rate constant at 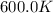 =
= 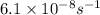
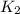 = rate constant at
= rate constant at 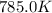 = ?
= ?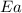 = activation energy for the reaction = 262 kJ/mole = 262000 J/mole
= activation energy for the reaction = 262 kJ/mole = 262000 J/mole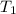 = initial temperature =
= initial temperature = 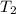 = final temperature =
= final temperature = ![\log (\frac{K_2}{6.1\times 10^{-8}s^{-1}})=\frac{262000J/mole}{2.303\times 8.314J/mole.K}[\frac{1}{600.0K}-\frac{1}{785.0K}]](/tpl/images/0147/7591/a9479.png)