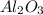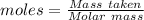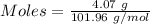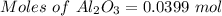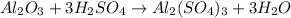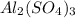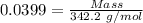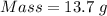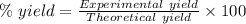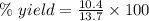Chemistry, 10.11.2019 03:31 babyboogrocks5572
For the following reaction, 4.07 grams of aluminum oxide are mixed with excess sulfuric acid. the reaction yields 10.4 grams of aluminum sulfate. aluminum oxide (s) + sulfuric acid (aq) aluminum sulfate (aq) + water (l) what is the theoretical yield of aluminum sulfate? grams what is the percent yield for this reaction? %

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Agas in a balloon at constant pressure has a volume of 160 ml at -125*c. what is its volume at 29.0*c?
Answers: 1

Chemistry, 22.06.2019 08:30
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 11:40
Effect of rotenone and antimycin a on electron transfer rotenone, a toxic natural product from plants, strongly inhibits nadh dehydrogenase of insect and fish mitochondria. antimycin a, a toxic antibiotic, strongly inhibits the oxidation of ubiquinol. (a) explain why rotenone ingestion is lethal to some insect and fish species. (b) explain why antimycin a is a poison. (c) given that rotenone and antimycin a are equally effective in blocking their respective sites in the electron-transfer chain, which would be a more potent poison? explain.
Answers: 3
You know the right answer?
For the following reaction, 4.07 grams of aluminum oxide are mixed with excess sulfuric acid. the re...
Questions

Biology, 17.10.2019 07:20


History, 17.10.2019 07:20

Mathematics, 17.10.2019 07:20

Computers and Technology, 17.10.2019 07:20


Mathematics, 17.10.2019 07:20



Mathematics, 17.10.2019 07:20


Mathematics, 17.10.2019 07:20

Mathematics, 17.10.2019 07:20

Biology, 17.10.2019 07:20

Physics, 17.10.2019 07:20




Mathematics, 17.10.2019 07:20