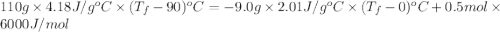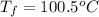Chemistry, 10.11.2019 03:31 julliette27
An ice cube of mass 9.0 g at temperature 0∘c is added to a cup of coffee, whose temperature is 90 ∘c and which contains 110 g of liquid. assume the specific heat capacity of the coffee is the same as that of water. the heat of fusion of ice (the heat associated with ice melting) is 6.0 kj/mol.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:30
An alcohol thermometer makes use of alcohol's changing in order to measure temperature. as the temperature goes up, the alcohol contained in the thermometer increases in volume, filling more of the thermometer's tube.
Answers: 3

Chemistry, 22.06.2019 02:00
In which of these cases are the two wave points considered to be in phase with each other?
Answers: 1

Chemistry, 22.06.2019 07:00
The blackbody curve for a star name zeta is shown below. what is the peak wavelength for this star ?
Answers: 1

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2
You know the right answer?
An ice cube of mass 9.0 g at temperature 0∘c is added to a cup of coffee, whose temperature is 90 ∘c...
Questions






Mathematics, 05.06.2020 18:00


English, 05.06.2020 18:00


Mathematics, 05.06.2020 18:00







History, 05.06.2020 18:00



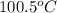
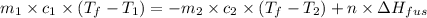
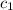 = specific heat of coffee = specific heat of water =
= specific heat of coffee = specific heat of water = 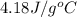 (as per question)
(as per question)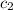 = specific heat of ice =
= specific heat of ice = 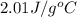
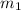 = mass of coffee = 110 g
= mass of coffee = 110 g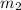 = mass of ice = 9.0 g
= mass of ice = 9.0 g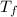 = final temperature = ?
= final temperature = ?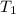 = initial temperature of coffee =
= initial temperature of coffee = 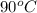
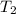 = initial temperature of ice =
= initial temperature of ice = 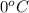
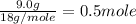
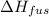 = 6.0 kJ/mol = 6000 J/mol
= 6.0 kJ/mol = 6000 J/mol