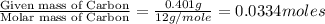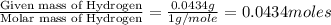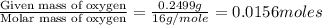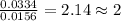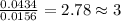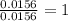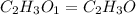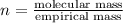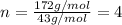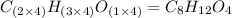Chemistry, 25.07.2019 03:10 paytonpaige22
When 0.6943 g of a compound is subjected to combustion analysis it produced 1.471 g co2 and 0.391 g h2o. what is its empirical and molecular formula if its molar mass is 172 g/mol if the compound is composed of only carbon, hydrogen, and oxygen.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be changed varied experimented controlled
Answers: 1

Chemistry, 22.06.2019 19:30
Helium decays to form lithium. which equation correctly describes this decay?
Answers: 2

Chemistry, 22.06.2019 22:30
Which of the following molecules is polar? c3h7oh c2h5cooh
Answers: 1

Chemistry, 23.06.2019 04:31
Which of the following is an example of how telecommunication devices people do their jobs? a.) a security guard checks the time using a digital watch. b.) a banker does some quick math using a solar calculator. c.) a nurse uses a digital thermometer to take a patient’s temperature. d.) a construction worker reports in to his office using a cell phone.
Answers: 1
You know the right answer?
When 0.6943 g of a compound is subjected to combustion analysis it produced 1.471 g co2 and 0.391 g...
Questions

Social Studies, 25.03.2021 19:30

Mathematics, 25.03.2021 19:30

Biology, 25.03.2021 19:30

Mathematics, 25.03.2021 19:30

English, 25.03.2021 19:30


Mathematics, 25.03.2021 19:30





Business, 25.03.2021 19:30





Mathematics, 25.03.2021 19:30



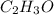 and
and 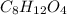 respectively.
respectively.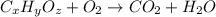
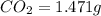
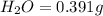
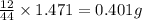 of carbon will be contained.
of carbon will be contained.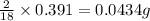 of hydrogen will be contained.
of hydrogen will be contained.