Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
How air particles exert a pressure on the inside of the balloon
Answers: 1

Chemistry, 22.06.2019 06:00
If you burn 10 kilograms of wood in a fire (combustion) what is the weight of the products after the fire has finished burning the wood?
Answers: 3

Chemistry, 22.06.2019 08:30
How does the principle of electromagnetism explain the interaction between earth’s magnetic field and the solar wind?
Answers: 1

Chemistry, 22.06.2019 19:20
15. which of the following is not human-caused groundwater pollution? a. water in an aquifer dissolves elements such as arsenic and mercury from surrounding rock. b. water in an aquifer is contaminated by leachate that seeps into the ground from a landfill. c. water in an aquifer becomes polluted with chemicals used in hydraulic fracturing, or fracking. d. water in an aquifer absorbs harmful bacteria from the drainage field of a septic tank.
Answers: 1
You know the right answer?
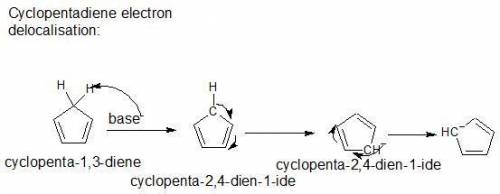
Cyclopentadiene is unusually acidic for a hydrocarbon. why? cyclopentadiene is aromatic. the conjug...
Questions

Biology, 18.03.2021 02:00

Arts, 18.03.2021 02:00


Mathematics, 18.03.2021 02:00

Social Studies, 18.03.2021 02:00

Chemistry, 18.03.2021 02:00


Mathematics, 18.03.2021 02:00

Mathematics, 18.03.2021 02:00

Mathematics, 18.03.2021 02:00






Mathematics, 18.03.2021 02:00

History, 18.03.2021 02:00


Health, 18.03.2021 02:00