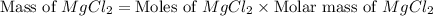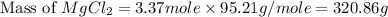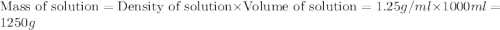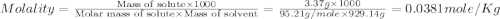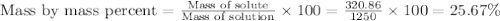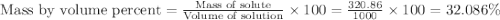Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Which element forms an ionic bond with flourine? 1) fluorine 2) carbon 3) potassium 4) oxygen
Answers: 1

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1

Chemistry, 22.06.2019 15:20
Select the most likely product for this reaction: koh(aq) + co2(g) – ? k2co3(aq) + h2o(1) k(s) + h2(g) + o2(g) k(s) + co3(9) +h2
Answers: 2

Chemistry, 22.06.2019 21:30
If you burn 46.6 g of hydrogen and produce 416 g of water, how much oxygen reacted
Answers: 3
You know the right answer?
The density of a 3.37m mgcl2 (fw = 95.21) is 1.25 g/ml. calulate the molality, mass/mass percent, an...
Questions










Social Studies, 03.01.2020 02:31








Social Studies, 03.01.2020 02:31


Biology, 03.01.2020 02:31

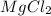 (solute) = 95.21 g/mole
(solute) = 95.21 g/mole