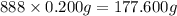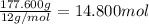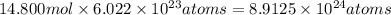The tiara worn by kate middleton for her wedding to prince william of england contains 888 diamonds and belongs to the british monarchy. if each diamond in the tiara is 1.0 carat, and given that diamond is a form of carbon and that 1 carat is defined as 0.200 g, calculate the number of atoms in the gemstones of that tiara.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 12:50
Which features are shown in the image? check all that apply. folds o anticlines o synclines o normal faults ostrike-slip faults
Answers: 1

Chemistry, 21.06.2019 19:30
The ph of carrots are 5.0 how it is classified a.acidic b.basic c.indicator d.neutral
Answers: 2

Chemistry, 22.06.2019 22:00
Ill give u brainliest pls how is mass of carbon conserved during cellular respiration
Answers: 1

You know the right answer?
The tiara worn by kate middleton for her wedding to prince william of england contains 888 diamonds...
Questions

Mathematics, 09.07.2019 08:30

Mathematics, 09.07.2019 08:30

Mathematics, 09.07.2019 08:30



Mathematics, 09.07.2019 08:30

Social Studies, 09.07.2019 08:30

History, 09.07.2019 08:30

Mathematics, 09.07.2019 08:30

Business, 09.07.2019 08:30

History, 09.07.2019 08:30

Mathematics, 09.07.2019 08:30

Mathematics, 09.07.2019 08:30

English, 09.07.2019 08:30

Mathematics, 09.07.2019 08:30


History, 09.07.2019 08:30

Mathematics, 09.07.2019 08:30


Biology, 09.07.2019 08:30

 .
.