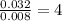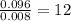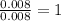Chemistry, 05.07.2019 18:20 hallmansean04
Asample containing only carbon, hydrogen, and silicon is subjected to elemental analysis. after complete combustion, a 0.7020 g sample of the compound yields 1.4 g of co2, 0.86 g of h2o, and 0.478 g of sio2. what is the empirical formula of the compound?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:00
The number to the right of an element's symbol (ex. c-12) identifies the of an isotope.
Answers: 1

Chemistry, 22.06.2019 14:50
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2


Chemistry, 23.06.2019 09:00
What properties would have caused early researchers to name hydrogen "inflammable air”
Answers: 3
You know the right answer?
Asample containing only carbon, hydrogen, and silicon is subjected to elemental analysis. after comp...
Questions





History, 20.03.2022 14:00

Mathematics, 20.03.2022 14:00

Mathematics, 20.03.2022 14:00







Mathematics, 20.03.2022 14:00


English, 20.03.2022 14:00

History, 20.03.2022 14:00



Mathematics, 20.03.2022 14:00

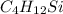 .
.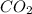 = 1.4 g
= 1.4 g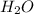 = 0.86 g
= 0.86 g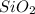 = 0.478 g
= 0.478 g 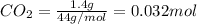
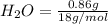 =0.048mol
=0.048mol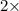 Moles of
Moles of 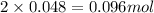
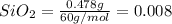 mol
mol