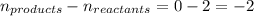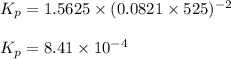Chemistry, 05.07.2019 18:20 aliceohern
Methanol can be synthesized from monoxide and hydrogen gas at 525 k. a reaction mixture consisting initially of 1.8 moles of co and 2.2 moles of h2 in 5.0-l container was found to contain 0.6 moles of ch3oh after reaching equilibrium (a) calculate equilibrium concentration (in molarity) of co and h2 (b) calculate equilibrium constants kc and kp for this reaction

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Which characteristics can be used to differentiate star systems? check all that apply.
Answers: 2

Chemistry, 22.06.2019 12:30
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1


Chemistry, 22.06.2019 21:00
Once similarity and one difference between a mixture of elements and a mixture of compounds
Answers: 3
You know the right answer?
Methanol can be synthesized from monoxide and hydrogen gas at 525 k. a reaction mixture consisting i...
Questions


French, 20.07.2019 12:00


French, 20.07.2019 12:00

Biology, 20.07.2019 12:00

History, 20.07.2019 12:00

Biology, 20.07.2019 12:00

Biology, 20.07.2019 12:00

Social Studies, 20.07.2019 12:00


Social Studies, 20.07.2019 12:00


History, 20.07.2019 12:00

Biology, 20.07.2019 12:00

Social Studies, 20.07.2019 12:00





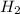 are 0.24 M and 0.32 M.
are 0.24 M and 0.32 M.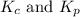 are 1.5625 and
are 1.5625 and 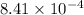
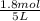
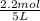
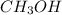 = 0.6
= 0.6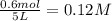
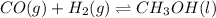
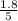
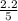 0
0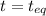
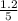
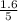
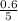
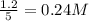
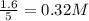
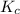 for the given chemical reaction follows:
for the given chemical reaction follows:![K_c=\frac{[CH_3OH]}{[CO][H_2]}](/tpl/images/0054/9747/63b2d.png)
![[CH_3OH]=0.12mol/L](/tpl/images/0054/9747/f965f.png)
![[CO]=0.24mol/L](/tpl/images/0054/9747/ee0b0.png)
![[H_2]=0.32mol/L](/tpl/images/0054/9747/aa037.png)
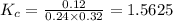
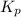 with
with 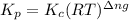
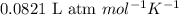
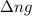 = change in number of moles of gas particles =
= change in number of moles of gas particles =