Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Acompound has a molar mass of 92.02 grams/mole, and its percent composition is 30.4% nitrogen (n) and 69.6% oxygen (o). what is its molecular formula? a. n2o4 b. no2 c. n2o d. n4o2
Answers: 1


Chemistry, 22.06.2019 23:00
What is the formula of the ionic compound composed of calcium cations and chloride anions
Answers: 1

Chemistry, 23.06.2019 01:00
If i had 2 m naoh solution, what does the 2 m stand for? 2 molar, but 2 of a solute in 1
Answers: 1
You know the right answer?
If 42.7 ml of a 0.208 m hcl solution is needed to neutralize a solution of ca(oh)2, how many grams o...
Questions




Physics, 09.12.2021 04:10











Biology, 09.12.2021 04:10


Computers and Technology, 09.12.2021 04:10

Biology, 09.12.2021 04:10

Biology, 09.12.2021 04:10

Biology, 09.12.2021 04:10

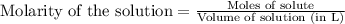
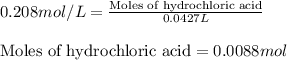
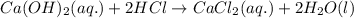
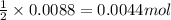 of calcium hydroxide.
of calcium hydroxide.