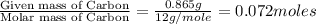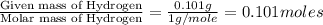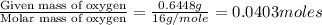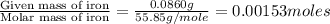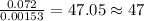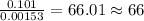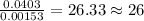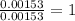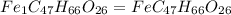Chemistry, 03.07.2019 21:20 delanieloya
When 1.6968 g of an organic iron compound containing fe, c, h, and o was burned in o2, 3.1737 g of co2 and 0.90829 g of h2o were produced. in a separate experiment to determine the mass percent of iron, 0.5446 g of the compound yielded 0.1230 g of fe2o3. what is the empirical formula of the compound?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Read these sentences from the text. near the equator, the tropics receive the most rain on a consistent basis. as a result, the fresh water falling into the ocean decrease the salinity of the surface water in that region. [. .] . . as the salt content of sea water increases, so does its density. what can you infer about how rain affects the density of surface water near the equator?
Answers: 1

Chemistry, 22.06.2019 03:40
Chemical kinetics what was the rate of reaction in trial 3? choose the closest answer.
Answers: 3

Chemistry, 22.06.2019 09:00
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium
Answers: 1
You know the right answer?
When 1.6968 g of an organic iron compound containing fe, c, h, and o was burned in o2, 3.1737 g of c...
Questions


Mathematics, 09.12.2020 22:50

Mathematics, 09.12.2020 22:50

Mathematics, 09.12.2020 22:50

Biology, 09.12.2020 22:50





Mathematics, 09.12.2020 22:50


Mathematics, 09.12.2020 22:50


Mathematics, 09.12.2020 22:50


Mathematics, 09.12.2020 22:50



Mathematics, 09.12.2020 22:50

Mathematics, 09.12.2020 22:50

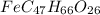
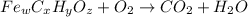
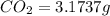
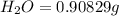
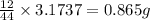 of carbon will be contained.
of carbon will be contained.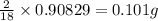 of hydrogen will be contained.
of hydrogen will be contained.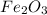 =
= 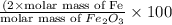
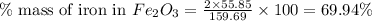
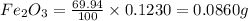 of iron.
of iron.