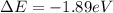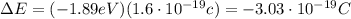Determine the energy change associated with the transition from n = 3 to n = 2 in the hydrogen atom. determine the energy change associated with the transition from n = 3 to n = 2 in the hydrogen atom. -1.82 × 10-19 j +2.69 × 10-19 j +5.51 × 10-19 j +3.03 × 10-19 j -3.03 × 10-19 j

Answers: 1


Another question on Physics


Physics, 22.06.2019 02:30
Agas initially at p1 = 1 bar and occupying a volume of 0.5 liter is compressed within a piston–cylinder assembly to a final pressure p2 = 4 bar. (a) if the relationship between pressure and volume during the compression is pv = constant, determine the volume, in liters, at a pressure of 3 bar. (b) repeat for a linear pressure–volume relationship between the same end states. reference
Answers: 1

Physics, 22.06.2019 05:00
Modern physics a photon emitted from an excited hydrogen atom has an energy of 3.02 electron volts. which electron energy-level transition would produce this photon? a. n=1 to n=6 b. n=2 to n=6 c. n=6 to n=1 d. n=6 to n=2 i chose b but the correct answer is d can someone tell me why? and what's the difference?
Answers: 1

You know the right answer?
Determine the energy change associated with the transition from n = 3 to n = 2 in the hydrogen atom....
Questions

Health, 19.05.2020 15:07

History, 19.05.2020 15:07

Mathematics, 19.05.2020 15:07

English, 19.05.2020 15:07



Mathematics, 19.05.2020 15:07

Mathematics, 19.05.2020 15:07

Mathematics, 19.05.2020 15:07

Mathematics, 19.05.2020 15:08

English, 19.05.2020 15:08

Mathematics, 19.05.2020 15:08

English, 19.05.2020 15:08

Mathematics, 19.05.2020 15:08

English, 19.05.2020 15:08



Mathematics, 19.05.2020 15:08

History, 19.05.2020 15:08

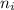 to a state
to a state 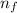 in a hydrogen atom is
in a hydrogen atom is![\Delta E=-13.6 Z^2 ( \frac{1}{n_f^2}- \frac{1}{n_i^2} )[eV]](/tpl/images/0148/1331/d3b72.png)
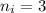 and
and 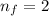 we find
we find