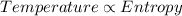Physics, 29.08.2019 00:30 kingkush85
Part a the third law of thermodynamics relates the entropy (randomness of matter to its absolute temperature. which of the following statements are in agreement with the third law of thermodynamics? check all that apply. check all that apply. at zero degrees kelvin, substances maximize entropy. at the melting point, the entropy of a system increases abruptly as the compound transforms into a liquid. as the temperature of a perfect crystal increases, the random vibrations of the molecules decrease. in a gaseous state, the entropy of the system is zero. in a crystalline state, there is a tendency to minimize entropy. at absolute zero, the interatomic distance within a crystal is minimized.

Answers: 2


Another question on Physics

Physics, 21.06.2019 18:20
What color of light must blue light mix with to create white light? a. yellow b. white c. blue d. green
Answers: 2

Physics, 21.06.2019 22:30
A1200 kg car traveling north at 10 m/s is rear-ended by a 2000 kg truck traveling at 30 m/s. what is the total momentum before and after the collision?
Answers: 3

Physics, 22.06.2019 02:00
The image shows a pendulum in simple harmonic motion the pendulum starts at a and swing to e
Answers: 1

Physics, 22.06.2019 18:30
How many meters will a car travel if its speed is 45 m/s in an interval of 11 seconds? question 2 options: a) 450 meters b) 495 meters c) 4.09 meters d) 498 meters
Answers: 2
You know the right answer?
Part a the third law of thermodynamics relates the entropy (randomness of matter to its absolute tem...
Questions













History, 28.10.2019 22:31