
In Parts B and C you saw that, according to Bohr's postulate, the electron radius r and the electron velocity v only have certain allowable values. Plug the values obtained for these two quantities into the energy statement given below (E=) to arrive at a new statement for the allowed energy levels in the Bohr atom. Express answer in terms of e, m, n, h, \epsilon _{0} . Equations:
v=nh/2mr\pi
r=n^{2}h^{2}\epsilon _{0}/m\pi e^{2}
E=\frac{1}{2}mv^{2}-(e^{2}/4\pi r\epsilon _{0})

Answers: 2


Another question on Physics

Physics, 21.06.2019 18:20
Find the vertex(use the completing the square method), findthe x- and y-intercepts, and sketch the graph offunction. y = 2x2 + 4x - 1
Answers: 3

Physics, 21.06.2019 22:30
Aforce of 200 n is applied to an input piston of cross-sectional area 2 sq. cm pushing it downward 2.8 cm. how far does the output piston of cross-sectional area 12 sq. cm move upward? show all work.
Answers: 1

Physics, 22.06.2019 11:30
Which of the following is the phase that results when the moon is on the opposite side of the earth from the sun? a. quarter moon b. crescent moon c. new moon d. full moon
Answers: 1

You know the right answer?
In Parts B and C you saw that, according to Bohr's postulate, the electron radius r and the electron...
Questions




History, 11.11.2020 18:00


Computers and Technology, 11.11.2020 18:00


English, 11.11.2020 18:00












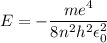
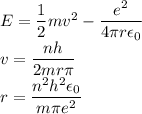
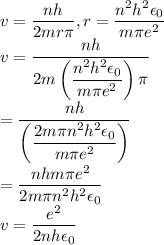
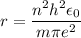
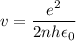
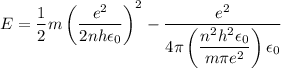
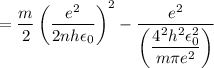
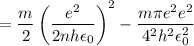
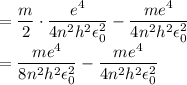
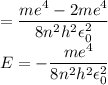
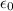 is:
is:

