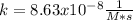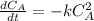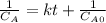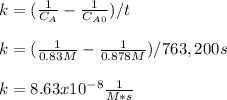Chemistry, 11.11.2020 18:00 rattler6125
The initial concentration of NOCl in the second-order reaction 2NOClâ2NO+Cl2 is 0.878M. After 763,200 seconds, the concentration of NOCl is 0.83M. What is the rate constant k for the reaction? Report your answer in scientific notation rounded to two significant figures. Use the multiplication symbol when reporting your answer rather than the letter x. Provide your answer below: $$ 1/M s

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:30
11. phosphorus-32 is radioactive and has a half life of 14 days. how much of a 124 mg sample of phosphorus-32 is present after 56 days? a) 7.75 mg b) 15.5 mg c) 31.0 mg d) 62.0 mg
Answers: 3


Chemistry, 22.06.2019 09:30
One way that radioactive waste is treated is by burying it in repositories. the repositories are found only in states with very low populations. true or false? a. trueb. false(also i meant to put high school but it put down middle school instead)
Answers: 1

Chemistry, 22.06.2019 21:20
The organs inside the body and how they function together
Answers: 3
You know the right answer?
The initial concentration of NOCl in the second-order reaction 2NOClâ2NO+Cl2 is 0.878M. After 763,20...
Questions

Computers and Technology, 04.03.2021 18:50

English, 04.03.2021 18:50

History, 04.03.2021 18:50

Mathematics, 04.03.2021 18:50

Social Studies, 04.03.2021 18:50



Arts, 04.03.2021 18:50


Mathematics, 04.03.2021 18:50

English, 04.03.2021 18:50







Mathematics, 04.03.2021 18:50

English, 04.03.2021 18:50

History, 04.03.2021 18:50