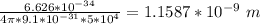Physics, 24.03.2020 22:58 miyocoilo07049
(a) Assume an electron in the ground state of the hydrogen atom moves at an average speed of 5.00 × 106 m/s. If the speed is known to an uncertainty of 1 percent, what is the minimum uncertainty in its position

Answers: 3


Another question on Physics

Physics, 21.06.2019 21:00
Mike walks 100 meter north, then walks 30 meters south. after this, he walks another 10 meters north. what is the magnitude of his total displacement during this walk,in meters?
Answers: 2

Physics, 21.06.2019 22:00
•• al and bert are jogging side-by-side on a trail in the woods at a speed of 0.75 m/s. suddenly al sees the end of the trail 35 m ahead and decides to speed up to reach it. he accelerates at a constant rate of 0.50 m/s2, while bert continues on at a constant speed. (a) how long does it take al to reach the end of the trail? (b) once he reaches the end of the trail, he immediately turns around and heads back along the trail with a constant speed of 0.85 m/s. how long does it take him to meet up with bert? (c) how far are they from the end of the trail when they meet?
Answers: 3

Physics, 22.06.2019 02:20
According to newton’s first law of motion, which force is expected to cause a body to accelerate?
Answers: 1

Physics, 22.06.2019 05:00
Red light strikes a metal surface and electrons are ejected. if violet light is now used with a 10% greater intensity, what will happen to the ejection rate (number of ejected electrons per second) and the maximum energy of the electrons? a) greater ejection rate; same maximum energyb) same ejection rate; greater maximum energyc) greater ejection rate; greater maximum energyd) same ejection rate; same maximum energye) none of the above answers are correct
Answers: 1
You know the right answer?
(a) Assume an electron in the ground state of the hydrogen atom moves at an average speed of 5.00 ×...
Questions


Chemistry, 26.05.2020 02:04

Mathematics, 26.05.2020 02:04



History, 26.05.2020 02:04













Geography, 26.05.2020 02:04