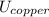Physics, 10.03.2020 04:37 eymurezgi1
A 50-kg iron block and a 20-kg copper block, both initially at 80°C, are dropped into a large lake at 15°C. Thermal equilibrium is established after a while as a result of heat transfer between the blocks and the lake water. Assuming the surroundings to be at 20°C, determine the amount of work that could have been produced if the entire process were executed in a reversible manner.

Answers: 1


Another question on Physics


Physics, 22.06.2019 10:30
The uniform slender bar rests on a smooth horizon- tal surface when a force f is applied normal to the bar at point a. point a is observed to have an initial acceleration aa of 20 m/s2, and the bar has a corre- sponding angular acceleration of 18 rad /s2. deter- mine the distance b.
Answers: 1

Physics, 22.06.2019 14:00
Amass attached to a spring is displaced from its equilibrium position by 5cm and released. the system then oscillates in simple harmonic motion with a period of 1s. if that same mass–spring system is displaced from equilibrium by 10cm instead, what will its period be in this case? a mass attached to a spring is displaced from its equilibrium position by and released. the system then oscillates in simple harmonic motion with a period of . if that same mass–spring system is displaced from equilibrium by instead, what will its period be in this case? a) 0.5sb) 2sc) 1sd) 1.4s
Answers: 2

Physics, 22.06.2019 14:30
An electromagnet is a device in which moving electric chargers in a coil of wire create a magnet.whats one advantage of using electromagnetic in devices
Answers: 1
You know the right answer?
A 50-kg iron block and a 20-kg copper block, both initially at 80°C, are dropped into a large lake a...
Questions

Health, 01.07.2019 18:30

Health, 01.07.2019 18:30




Health, 01.07.2019 18:30


Health, 01.07.2019 18:30


Health, 01.07.2019 18:30

Health, 01.07.2019 18:30

Health, 01.07.2019 18:30

Health, 01.07.2019 18:30


Mathematics, 01.07.2019 18:30


Mathematics, 01.07.2019 18:30

Health, 01.07.2019 18:30


Health, 01.07.2019 18:30

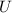 = Δ
= Δ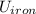 + Δ
+ Δ