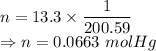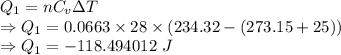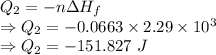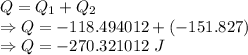Physics, 21.01.2020 03:31 haftjnd9156
Calculate the heat energy released when 13.3 g of liquid mercury at 25.00 c is converted to solid mercury at its melting point. constants for mercury at 1 atmheat capacity of hg(l) 28.0 j/(mol k)melting point 234.32 kenthalphy of fusion 2.29 kj/mol

Answers: 1


Another question on Physics

Physics, 21.06.2019 16:00
Your pendulum clock which advances 1.0 s for every complete oscillation of the pendulum is running perfectly on earth at a site where the magnitude of the acceleration due to gravity is 9.80 m/s2. you send the clock to a location on the moon where the magnitude of the acceleration due to gravity is 1.65 m/s2.
Answers: 2

Physics, 22.06.2019 09:30
An electric clothes dryer has a resistance of 8 ohms. it draws 30 a of current. what is the voltage, in volts, of the wall outlet that it is plugged into?
Answers: 2


Physics, 22.06.2019 12:20
The diagram shows four locations in the electric field of a positive point charge m. at which location is the electric potential the greatest.
Answers: 2
You know the right answer?
Calculate the heat energy released when 13.3 g of liquid mercury at 25.00 c is converted to solid me...
Questions




Mathematics, 11.10.2019 22:40






Mathematics, 11.10.2019 22:40

English, 11.10.2019 22:40









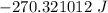
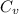 = Heat capacity of Hg = 28 J/mol
= Heat capacity of Hg = 28 J/mol = Change in temperature =
= Change in temperature = 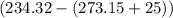
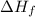 = Enthalpy of fusion = 2.29 kJ/mol
= Enthalpy of fusion = 2.29 kJ/mol