
Physics, 28.11.2019 22:31 vegeta8375
A4.6-kg block of ice originally at 263 k is placed in thermal contact with a 15.7-kg block of silver (cag = 233 j/kg-k) which is initially at 1052 k. the h2o - silver system is insulated so no heat flows into or out of it. at what temperature will the system achieve equilibrium?

Answers: 1


Another question on Physics

Physics, 22.06.2019 08:30
Does anyone know how to solve this problem? i really need . i made an attempt but i just cant get it. a metal rod is 25.000 cm long at 25.0 degrees celsius. when heated to 102.0 degrees celsius, it is 25.054 cm long. what is the coefficient of linear expansion for this metal.
Answers: 3

Physics, 22.06.2019 09:30
Acar traveling at 22 m/s starts to decelerate steadily. it comes to a complete stop in 15 seconds. what is it’s acceleration?
Answers: 1

Physics, 22.06.2019 18:30
Asmall 12.00g plastic ball is suspended by a string in a uniform, horizontal electric field with a magnitude of 10^3 n/c. if the ball is in equilibrium when the string makes a 30 ° angle with the vertical, what is the net charge on the ball?
Answers: 1

Physics, 22.06.2019 18:30
Examples of states of consciousness include a. daydreaming b. dreaming during sleep c. hypnosis d. all of the above
Answers: 2
You know the right answer?
A4.6-kg block of ice originally at 263 k is placed in thermal contact with a 15.7-kg block of silver...
Questions


Chemistry, 01.07.2020 15:01


Physics, 01.07.2020 15:01


Mathematics, 01.07.2020 15:01






Physics, 01.07.2020 15:01








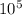 J/kg
J/kg

