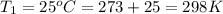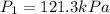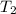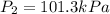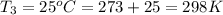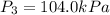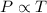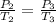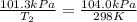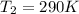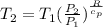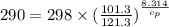Physics, 12.11.2019 02:31 jackiecroce1
Calculate the heat capacity of a gas sample from the following information: the sam- ple comes to equilibrium in a flask at 25°c and 121.3 kpa. a stopcock is opened briefly, allowing the pressure to drop to 101.3 kpa. with the stopcock closed, the flask warms, returning to 25°c, and the pressure is measured as 104.0 kpa. determine cp in j·mol−1·k−1 assuming the gas to be ideal and the expansion of the gas remaining in the flask to be reversible and adiabatic.

Answers: 3


Another question on Physics

Physics, 22.06.2019 04:20
Astone is thrown into a pond. what happens to the amplitude of the resulting waves as they get farther from the point where the stone hit the water? explain.
Answers: 3

Physics, 22.06.2019 09:30
True or false graphs must include scales that increase by the same amount
Answers: 3


Physics, 22.06.2019 11:30
Which of the following is the phase that results when the moon is on the opposite side of the earth from the sun? a. quarter moon b. crescent moon c. new moon d. full moon
Answers: 1
You know the right answer?
Calculate the heat capacity of a gas sample from the following information: the sam- ple comes to e...
Questions

Mathematics, 01.08.2020 14:01





History, 01.08.2020 14:01


Mathematics, 01.08.2020 14:01

English, 01.08.2020 15:01


Mathematics, 01.08.2020 15:01

Mathematics, 01.08.2020 15:01



English, 01.08.2020 15:01

Mathematics, 01.08.2020 15:01

Computers and Technology, 01.08.2020 15:01

Physics, 01.08.2020 15:01


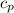 for reversible and adiabatic expansion is 55.04 J/mol.K
for reversible and adiabatic expansion is 55.04 J/mol.K