
A25.0-g sample of copper at 363 k is placed in i 00.0 g of water at 293 k. the copper and water quickly come to the sa me temperature by the process of heat transfer from copper to water. calculate the final temperature of the water. the molar heat capacity of copper is 24.5 j · k-1 ·mol-1 and that of water is 75.2 1 · k -1 -mol-1•

Answers: 2


Another question on Physics

Physics, 21.06.2019 16:10
For a particular reaction, ? h = -144.6 kj and ? s = -301.2 j/k. calculate ? g for this reaction at 298 k.
Answers: 1

Physics, 21.06.2019 17:30
Abatter hits the baseball a with an initial velocity of v0 = 110 ft/sec directly toward fielder b at an angle of 23° to the horizontal; the initial position of the ball is 2.2 ft above ground level. fielder b requires 0.44 sec to judge where the ball should be caught and begins moving to that position with constant speed. because of great experience, fielder b chooses his running speed so that he arrives at the “catch position” simultaneously with the baseball. the catch position is the field location at which the ball altitude is 8.4 ft. determine the velocity of the ball relative to the fielder at the instant the catch is made.
Answers: 1

Physics, 21.06.2019 18:40
Carbon-14 is a radioactive element that undergoes beta decay. which force is responsible for allowing carbon-14 to become stable? electromagnetic gravitational weak nuclear strong nuclear
Answers: 3

Physics, 21.06.2019 23:00
For general projectile motion, when the projectile is at the highest point of its trajectory, its acceleration is zero? a)the horizontal and vertical components of its velocity are zero. b) its velocity is perpendicular to the acceleration. c)the horizontal component of its velocity is zero. d)its velocity and acceleration are both zero.
Answers: 1
You know the right answer?
A25.0-g sample of copper at 363 k is placed in i 00.0 g of water at 293 k. the copper and water quic...
Questions

Health, 26.03.2020 01:00

Social Studies, 26.03.2020 01:00


Mathematics, 26.03.2020 01:00










Chemistry, 26.03.2020 01:00



Mathematics, 26.03.2020 01:00



Mathematics, 26.03.2020 01:00

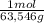 ×mass×ΔT
×mass×ΔT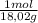 ×mass×ΔT
×mass×ΔT


