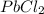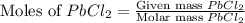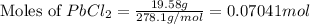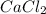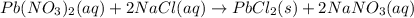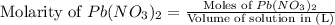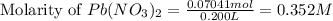Chemistry, 26.03.2020 01:00 zahnjoey4661
A solution of NaCl ( aq ) is added slowly to a solution of lead nitrate, Pb ( NO 3 ) 2 ( aq ) , until no further precipitation occurs. The precipitate is collected by filtration, dried, and weighed. A total of 19.58 g CaCl2 ( s ) is obtained from 200.0 mL of the original solution. Calculate the molarity of the Pb ( NO 3 ) 2 ( aq ) solution.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 23:10
Afusion reaction takes place between carbon and another element. neutrons are released, and a different element is formed. the different element is a) lighter than helium.b)heavier than helium.c)the same weight as helium.d)dependent on the element that reacted with carbon.
Answers: 3

Chemistry, 22.06.2019 23:30
If it is an isoelectronic series select true, if not select false. o2-, s2-, se2-, te2- na+, k+, rb+, cs+ n3-, p3-, as3-, sb3- ag, cd+, sn3+, sb4+ f-, cl-, br-, i- f-, ne, na+, mg2+ s2-, s, s6+
Answers: 1

Chemistry, 23.06.2019 00:00
This statement about matter and its behavior is best classified as a
Answers: 1
You know the right answer?
A solution of NaCl ( aq ) is added slowly to a solution of lead nitrate, Pb ( NO 3 ) 2 ( aq ) , unti...
Questions

Arts, 04.02.2021 14:00

Mathematics, 04.02.2021 14:00


Mathematics, 04.02.2021 14:00

English, 04.02.2021 14:00

Mathematics, 04.02.2021 14:00

Mathematics, 04.02.2021 14:00

Mathematics, 04.02.2021 14:00


Mathematics, 04.02.2021 14:00




Mathematics, 04.02.2021 14:00


English, 04.02.2021 14:00

Mathematics, 04.02.2021 14:00

Mathematics, 04.02.2021 14:00

Social Studies, 04.02.2021 14:00

Chemistry, 04.02.2021 14:00

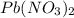 solution is, 0.352 M
solution is, 0.352 M