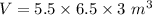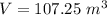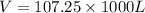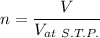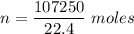Physics, 09.10.2019 02:20 mullery7482
Th e heat capacity of air is much smaller than that of water, and relatively modest amounts of heat are needed to change its temperature. th is is one of the reasons why desert regions, although very hot during the day, are bitterly cold at night. th e heat capacity of air at room temperature and pressure is approximately 21 j k−1 mol−1. how much energy is required to raise the temperature of a room of dimensions 5.5 m × 6.5 m × 3.0 m by 10°c? if losses are neglected, how long will it take a heater rated at 1.5 kw to achieve that increase given that 1 w = 1 j s−1?

Answers: 1


Another question on Physics

Physics, 22.06.2019 05:30
If gases like carbon dioxide and methane make up less than 1% of the total atmosphere, why is it important for scientists to monitor changes in percentages of these gases?
Answers: 1

Physics, 22.06.2019 07:00
Suppose while hauling rocks, you accidentally drop one. it breaks apart in flat, planar sections. what type of rock did you just drop? 20 points
Answers: 3

Physics, 22.06.2019 12:50
The vapour pressure of benzene is 53.3 kpa at 60.6 °c, but it fell to 51.5 kpa when 19.0 g of a non-volatile organic compound was dissolved in 500 g of benzene. calculate the molar mass of the compound.
Answers: 2

Physics, 22.06.2019 17:00
How much energy is supplied to each coulomb of charge that flows through a 12-v battery?
Answers: 1
You know the right answer?
Th e heat capacity of air is much smaller than that of water, and relatively modest amounts of heat...
Questions



Advanced Placement (AP), 29.07.2019 01:00




Biology, 29.07.2019 01:00


Social Studies, 29.07.2019 01:00






Health, 29.07.2019 01:00

History, 29.07.2019 01:00

Mathematics, 29.07.2019 01:00


History, 29.07.2019 01:00