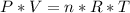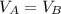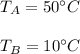Physics, 06.10.2019 01:30 parminder44
We have two equal size boxes, a and b. each box contains gas that behaves as an ideal gas. we insert a thermometer into each box and find that the gas in box s is at a temperature of 50°c while that in b is at 10°c. this is all we know about the gas in the boxes. which of the following statements must be true? which could be true? explain the reasoning behind your answers.
a) the pressure in a is higher than in b
b) there are more molecules in a than in b
c) a and b cannot contain the same type of gas
d) the molecules in a have more average kinetic energy per molecule than those in b
e) the molecules in a are moving faster than those in b

Answers: 2


Another question on Physics

Physics, 22.06.2019 13:40
An ideal otto cycle has a compression ratio of 10.5, takes in air at 90 kpa and 40°c, and is repeated 2500 times per minute. using constant specific heats at room temperature, determine the thermal efficiency of this cycle and the rate of heat input if the cycle is to produce 90 kw of power.
Answers: 2

Physics, 22.06.2019 16:30
Each neuron shown in this figure innervates a group of muscle fibers. what is the term for a group of muscle fibers innervated by a single neuron?
Answers: 1

Physics, 22.06.2019 17:20
Properties seen when one one substance changes to another are known as properties
Answers: 1

Physics, 22.06.2019 23:30
The photo above shows oil and vinegar in a pitcher. the top make a claim about about the density of the vinegar compared to the density of the oil summarize the evidence to support the claim and explain your reasoning
Answers: 3
You know the right answer?
We have two equal size boxes, a and b. each box contains gas that behaves as an ideal gas. we insert...
Questions







Health, 20.10.2019 16:00

Spanish, 20.10.2019 16:00

Chemistry, 20.10.2019 16:00

Biology, 20.10.2019 16:00

History, 20.10.2019 16:00

History, 20.10.2019 16:00

Mathematics, 20.10.2019 16:00