
Physics, 21.08.2019 04:00 veronicacalyn
An isotope of californium-252 undergoes a spontaneous fission, producing cesium-135, 3 neutrons, and one other isotope. a) determine the identity of the other isotope produced in the fission, and write out the reaction equation, using full isotopic notation, for example pm → c022 + seag + 2n. b) calculate the amount of mass (in u) lost during this fission. the masses of the three isotopes are: 252.081 626 u (californium), 134.905 978 u (cesium), 113.935 880 u (unknown). maintain a precision of no less than 7 sf as you work. c) calculate the energy (in mev) released by this fission, accurate to 3 sf.

Answers: 1


Another question on Physics

Physics, 22.06.2019 05:30
Acombination reaction is when two or more combine to form one product. a decomposition reaction is when a substance breaks down into two or more simpler substances in a chemical reaction.
Answers: 1


Physics, 22.06.2019 19:00
The direction of the current in an alternating current circuit remains constant. is direct. changes repeatedly. changes randomly.
Answers: 1

Physics, 22.06.2019 22:00
Should we celebrate the voyages of zheng he mini q answer key
Answers: 3
You know the right answer?
An isotope of californium-252 undergoes a spontaneous fission, producing cesium-135, 3 neutrons, and...
Questions



English, 23.10.2020 16:00






Law, 23.10.2020 16:00










Mathematics, 23.10.2020 16:10

Mathematics, 23.10.2020 16:10

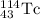
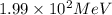
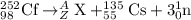
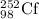 = 252.081626 u
= 252.081626 u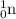 = 1.008665 u
= 1.008665 u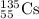 = 134.905978 u
= 134.905978 u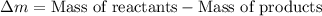
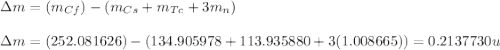
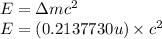
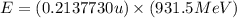 (Conversion factor:
(Conversion factor: 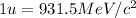 )
)


