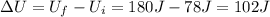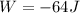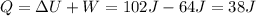Physics, 15.07.2019 09:00 bluetigerbird5323
Jin knows that the initial internal energy of a closed system is 78 j and the final internal energy is 180 j. he also knows that 64 j of energy are used to do work. to find the heat added to the system, jin completes the steps below. 1. add the initial internal energy plus the final internal energy to find the change in internal energy. 2. add the change in internal energy to the energy used to do work. 3. write the answer in joules.

Answers: 1


Another question on Physics

Physics, 21.06.2019 20:40
If an object with an initial temperature of 300 k increases its temperature by 1°c every minute, by how many degrees fahrenheit will its temperature have increased in 10 minutes? (a) 6°f (b) 10°f (c) 18°f (d) 30°f
Answers: 3

Physics, 21.06.2019 23:00
Atrain departs from its station at a constant acceleration of 5 m/s. what is the speed of the train at the end of 20s?
Answers: 1

Physics, 22.06.2019 06:30
Air initially at 0.75 bar, 1000 k, and occupying a volume of 0.12 m^3 undergoes two processes. process 1-2: the air is compressed isothermally until the volume is halved. process 2-3: the air undergoes a constant pressure process until the volume is halved again. assume ideal gas behavior. a) determine the mass of the air, in kg. b) the work and the heat transfer for each of the two processes, in kj. (100 kj = 1 bar . m^3)
Answers: 1

Physics, 22.06.2019 11:00
Which sound characteristic is not affected by the relative motion of an object
Answers: 2
You know the right answer?
Jin knows that the initial internal energy of a closed system is 78 j and the final internal energy...
Questions

History, 16.09.2019 22:10


Mathematics, 16.09.2019 22:10


History, 16.09.2019 22:10



History, 16.09.2019 22:10

Chemistry, 16.09.2019 22:10




Mathematics, 16.09.2019 22:10

Social Studies, 16.09.2019 22:10


Law, 16.09.2019 22:10



Mathematics, 16.09.2019 22:10

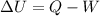 (1)
(1)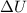 is the variation of internal energy
is the variation of internal energy