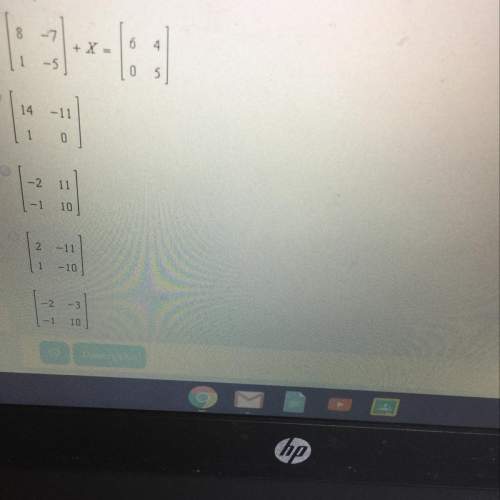
Mathematics, 16.02.2021 06:20 iixyloa
The gas law for an ideal gas at absolute temperature T (in kelvins), pressure P (in atmospheres), and volume V (in liters) is PV = nRT, where n is the number of moles of the gas and R = 0.0821 is the gas constant. Suppose that, at a certain instant, P = 8.0 atm and is increasing at a rate of 0.14 atm/min and V = 14 L and is decreasing at a rate of 0.16 L/min. Find the rate of change of T with respect to time (in K/min) at that instant if n = 10 mol. (Round your answer to four decimal places.) dT dt = 3.78 K/min

Answers: 1


Another question on Mathematics

Mathematics, 21.06.2019 20:00
If private savings 'v' = 0.75s and total savings 's' equals $4.20 billion, solve for public and private savings.
Answers: 2

Mathematics, 21.06.2019 20:40
In each of the cases that follow, the magnitude of a vector is given along with the counterclockwise angle it makes with the +x axis. use trigonometry to find the x and y components of the vector. also, sketch each vector approximately to scale to see if your calculated answers seem reasonable. (a) 50.0 n at 60.0°, (b) 75 m/ s at 5π/ 6 rad, (c) 254 lb at 325°, (d) 69 km at 1.1π rad.
Answers: 3

Mathematics, 22.06.2019 02:00
If the angle bisectors of a pair of opposite angles of a quadrilateral are the opposite sides of a parallelogram formed by the two angle bisectors and two sides of the quadrilateral, is the quadrilateral always a parallelogram? explain your answer.
Answers: 3

You know the right answer?
The gas law for an ideal gas at absolute temperature T (in kelvins), pressure P (in atmospheres), an...
Questions

Mathematics, 01.12.2020 03:00




Computers and Technology, 01.12.2020 03:00





Mathematics, 01.12.2020 03:00




Mathematics, 01.12.2020 03:00

History, 01.12.2020 03:00


Mathematics, 01.12.2020 03:00

Mathematics, 01.12.2020 03:00