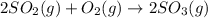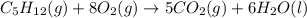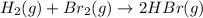Chemistry, 01.12.2020 03:00 AlaskaAirlines
At constant pressure for which of the reactions shown below should Delta H be greater than Delta E°?
1.2 SO2(g) + O2(g) - 2 SO3(g)
II. C5H12(9) + 8 O2(g) - 5 CO2(g) + 6 H20(1)
III. H2(g) + Br2(g) → 2 HBr(9),
IV. N204(9) - 2 NO2(g)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
How did planetesmals form planets? a. they broke apart into smaller chunks.b. they collided and stuck together.c. they cooled and pulled ice together.d. they began to rotate.
Answers: 1

Chemistry, 22.06.2019 07:00
How far is the region from the equator or control climate
Answers: 1

Chemistry, 22.06.2019 07:40
The formation of a solid, also known as a is an indication of a chemical change. precipitate particulate particle powder
Answers: 3

Chemistry, 22.06.2019 14:00
650.j is the same amount of energy as? 2720cal1550cal650.cal2.72cal
Answers: 2
You know the right answer?
At constant pressure for which of the reactions shown below should Delta H be greater than Delta E°?...
Questions

Mathematics, 27.03.2020 19:31


Computers and Technology, 27.03.2020 19:31


Chemistry, 27.03.2020 19:31

Mathematics, 27.03.2020 19:31


Mathematics, 27.03.2020 19:31

Mathematics, 27.03.2020 19:31

History, 27.03.2020 19:31


Mathematics, 27.03.2020 19:31

History, 27.03.2020 19:31

Mathematics, 27.03.2020 19:31

Biology, 27.03.2020 19:31


History, 27.03.2020 19:31

Mathematics, 27.03.2020 19:31

Health, 27.03.2020 19:31

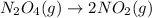 has higher value of
has higher value of 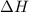 than
than 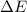
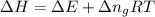
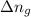 = change in the gaseous moles of the reaction = Moles of product - Moles of reactant
= change in the gaseous moles of the reaction = Moles of product - Moles of reactant