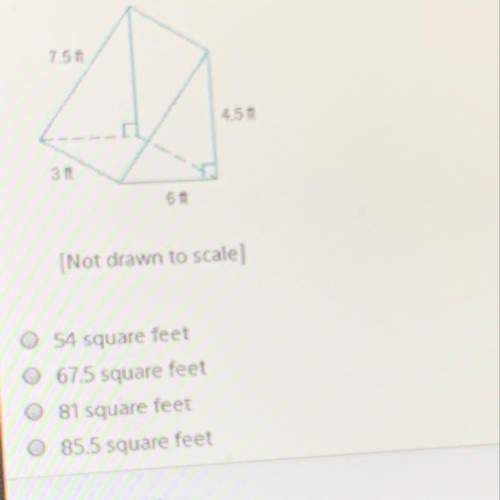
Mathematics, 17.10.2019 04:30 elizabethhubbe
Suppose that stoichiometric amounts of nitrogen gas and hydrogen gas react in a calorimeter to produce 5.00 g of ammonia gas. the calorimeter temperature rises 0.42ác. the calorimeter and water have a combined heat capacity of 32.16 kj/k. calculate the heat of formation of ammonia, _háf, in kj/mol. the formation reaction for ammonia is: 0.5n2(g) + 1.5h2(g) _ nh3(g).

Answers: 1


Another question on Mathematics

Mathematics, 21.06.2019 15:30
The area of a rectangle is 15+5y square feet. which of the following expressions could be the length of the sides of the rectangle, in feet a. 5 and 3y b. 5 and 3+y c. 5 and 5+y d. 3 and 5+3y
Answers: 1

Mathematics, 21.06.2019 16:20
7.(03.01 lc)which set represents the range of the function shown? {(-1, 5), (2,8), (5, 3), 13, -4)} (5 points){-1, 2, 5, 13){(5, -1), (8, 2), (3,5), (-4, 13)){-4, 3, 5, 8}{-4, -1, 2, 3, 5, 5, 8, 13}
Answers: 3

Mathematics, 21.06.2019 18:00
What is the location of point g, which partitions the directed line segment from d to f into a 5: 4 ratio? –1 0 2 3
Answers: 1

You know the right answer?
Suppose that stoichiometric amounts of nitrogen gas and hydrogen gas react in a calorimeter to produ...
Questions















Mathematics, 15.04.2020 05:33

Physics, 15.04.2020 05:33



Law, 15.04.2020 05:33