
Mathematics, 26.02.2020 00:15 josuemartinez1030
Calculate the specific heat of a metal (in calories/gram-degree C) from the following data. A container made of the metal has a mass of 3.77 kg and contains 18.1 kg of water. A 1.45 kg piece of the same metal, initially at a temperature of 164 degrees C, is placed in the water. The container and water initially have a temperature of 15 degrees C, and the final temperature of the entire system is 18 degrees C.

Answers: 3


Another question on Mathematics


Mathematics, 21.06.2019 19:00
You and a friend went to the movies.you spent half your money on tickets. then you spent 1/4 of the money on popcorn, $2 on candy, and $3 on a soda. if you have $1 left, hiw much money did u take to the movies?
Answers: 1

Mathematics, 22.06.2019 00:20
20 ! need ! which exponential function is represented by the table? f(x) = 0.2(0.5x)f(x) = 0.5(5x)f(x) = 0.5(0.2x)f(x) = 0.2(0.2x)
Answers: 1

You know the right answer?
Calculate the specific heat of a metal (in calories/gram-degree C) from the following data. A contai...
Questions

Health, 02.02.2021 03:10


Mathematics, 02.02.2021 03:10

History, 02.02.2021 03:10

Mathematics, 02.02.2021 03:10


Mathematics, 02.02.2021 03:10

Mathematics, 02.02.2021 03:10

English, 02.02.2021 03:10


Mathematics, 02.02.2021 03:10





English, 02.02.2021 03:20



History, 02.02.2021 03:20

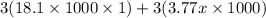 .
.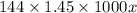 .
.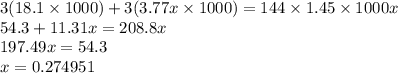 .
.


