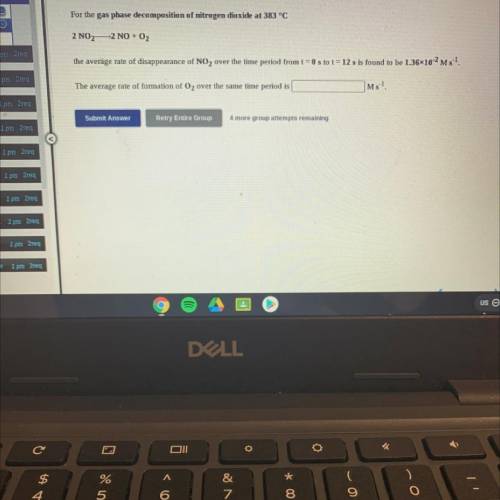
For the gas phase decomposition of nitrogen dioxide at 383 °C
2 NO2—2 NO + O2
the average rat...

Chemistry, 02.02.2021 03:20 tyneshiajones124
For the gas phase decomposition of nitrogen dioxide at 383 °C
2 NO2—2 NO + O2
the average rate of disappearance of NO2 over the time period from t = 0 s to t = 12 s is found to be 1.3610-2 Ms 1.
The average rate of formation of O2 over the same time period is
Ms-1

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Which of the following would have less momentum than a 52 kg cheetah running at 10 m/s?
Answers: 2

Chemistry, 22.06.2019 09:20
Sugar is dissolved in water. which is the solute? sugar neither both water
Answers: 1

Chemistry, 22.06.2019 10:00
Diffraction is when light is bent around obstructions. which of the these observation about clouds would indicate diffraction? a) after rain storms, you can sometimes see rainbows. b) clouds are white or gray and cannot be seen through. c) on a cloudy day, the temperature tends to be cooler than a sunny day. d) the edges of dark clouds appear lighter. this
Answers: 3

Chemistry, 22.06.2019 10:00
Drug abuse will not lead to physical and psychological dependence. true or false ?
Answers: 2
You know the right answer?
Questions

History, 23.03.2021 05:20

History, 23.03.2021 05:20


English, 23.03.2021 05:20



Mathematics, 23.03.2021 05:20



Biology, 23.03.2021 05:20

History, 23.03.2021 05:20

English, 23.03.2021 05:20


Physics, 23.03.2021 05:20



Mathematics, 23.03.2021 05:20

Business, 23.03.2021 05:20

Biology, 23.03.2021 05:20



