Engineering, 18.12.2019 00:31 julianastri6841
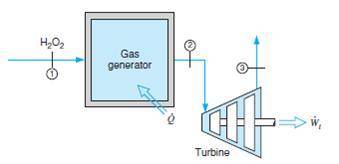
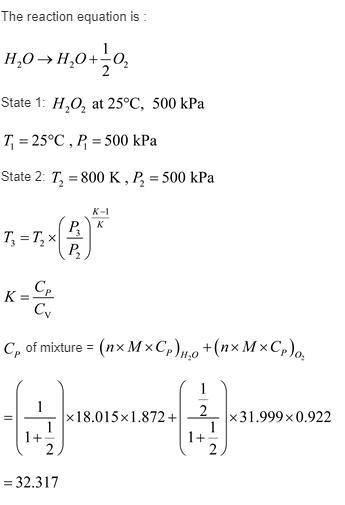
Hydrogen peroxide, h2o2, enters a gas generator at 25 celsius, 500 kpa, at the rate of 0.1 kg/s and is decomposed to steam and oxygen exiting at 800 k, 500 kpa. the resulting mixture is expanded through a turbine to atmospheric pressure, 100 kpa. determine the power output of the turbine and the heat transfer rate in the gas generator. the enthalpy of formation of liquid h2o2 is −187 583 kj/kmol.

Answers: 1


Another question on Engineering

Engineering, 04.07.2019 19:10
With increases in magnification, which of the following occur? a. the field of view decreases. b. the ambient illumination decreases. c. the larger parts can be measured. d. the eyepiece must be raised.
Answers: 1

Engineering, 04.07.2019 19:20
Liquid flows at steady state at a rate of 2 lb/'s through a pump, which operates to raise the elevation of the liquid 100 ft from control volume inlet to exit. the liquid specific enthalpy at the inlet is 40.09 btu/lb and at the exit is 40.94 btub. the pump requires 3 btu/s of power to operate. if kinetic energy effects are negligible and gravitational acceleration is 32.174 tt/s, the heat transfer rate associated with this steady state process is most closely 1)-2,02 btu/s from the liquid to the surroundings 2)-3.98 btu/s from the surroundings to the liquid. 3)-4.96 btu/s from the surroundings to the liquid. 4)-1.04 btu/s from the liquid to the surroundings.
Answers: 2

Engineering, 06.07.2019 03:10
Consider two concentric spheres forming an enclosure with diameters of 12 cm and 18 cm the spheres are maintained at uniform temperatures ti-50°c and t2 = 250°c and have emissivities .45 and .8, respectively. determine the net rate of radiation heat transfer between the two spheres per unit surface area.
Answers: 1

Engineering, 06.07.2019 03:20
Steam at a pressure of 100 kpa and a quality of 50% initially fills a rigid vessel having a volume of 0.5 m^3. the steam is then heated, causing the pressure in the vessel to rise to 150 kpa. determine: i. the mass of the steam in the vessel. ii. the temperature and quality of the steam after the heating process. ii the mass of the vapour, mg and liquid, m in the vessel after the heating process. if the steam in the vessel is now further heated, what would the pressure and temperature in the vessel be when all steam has turned into saturated vapour? b. sketch the processes in part (a) and part (b) on p-v and t-v diagrams, indicating clearly the temperatures, pressures and the paths. c. (0.59kg, 111.4°c, 73%, 0.4307kg, 0.1 593kg, 2.11 bar, 121.84°c)
Answers: 1
You know the right answer?
Hydrogen peroxide, h2o2, enters a gas generator at 25 celsius, 500 kpa, at the rate of 0.1 kg/s and...
Questions

Mathematics, 24.10.2019 06:43

Physics, 24.10.2019 06:43

Biology, 24.10.2019 06:43

Mathematics, 24.10.2019 06:43







Business, 24.10.2019 06:43


History, 24.10.2019 06:43

Arts, 24.10.2019 06:43

Mathematics, 24.10.2019 06:43


Biology, 24.10.2019 06:43

Health, 24.10.2019 06:43