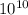Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:50
Compare the equilibrium constants for the systems shown in the table. which favors products the most? which favors products the least? rank these systems in order from most to least in terms of favoring products rather than reactants. d > b > a > c c > a > b > d b > c > d > a a > d > c > b
Answers: 1

Chemistry, 22.06.2019 09:00
The diagram below shows a cell placed in a solution.a cell is shown placed inside a beaker. it is labeled cell. the solution inside the beaker is labeled 40% salt solution and the solution inside the cell is labeled 20% salt solution.only water is allowed to move in and out of the cell. what will most likely happen to the cell? it will expand as water moves out of it. it will shrink as water moves out of it.it will expand as water moves into it. it will shrink as water moves into it.
Answers: 2


Chemistry, 23.06.2019 01:00
If a sample of radioactive isotopes takes 600 minutes to decay from 400 grams to 50 grams, what is the half-life of the isotope?
Answers: 1
You know the right answer?
Calculate the equilibrium constant for the reaction between fe2+(aq) and zn(s) under standard condit...
Questions


Chemistry, 05.06.2020 10:59

Mathematics, 05.06.2020 10:59




Spanish, 05.06.2020 10:59


Chemistry, 05.06.2020 10:59

Chemistry, 05.06.2020 10:59

Mathematics, 05.06.2020 10:59

Mathematics, 05.06.2020 10:59


Physics, 05.06.2020 10:59

Mathematics, 05.06.2020 10:59




Physics, 05.06.2020 11:57

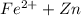 → Fe +
→ Fe + 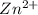
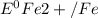 = standard reduction potential of Fe2+/Fe = -0.44 v
= standard reduction potential of Fe2+/Fe = -0.44 v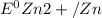 = standard reduction potential of Zn2+/Zn = -0.763 v
= standard reduction potential of Zn2+/Zn = -0.763 v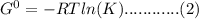
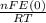 )= exp (
)= exp (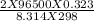 ) = 8.46 x
) = 8.46 x