
Chemistry, 21.07.2019 23:00 dajeourcrazy15
A5.018 gram sample of a certain hydrate of magnesium sulfate, mgso4•xh2o, is heated until all the water is driven off. the resulting anhydrous compound weighs 2.449 grams. what is the formula of the hydrate?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
For each of the following types of reactions, write a general reaction formula in the symbolic form—for example, a + b → ab. single-displacement double-displacement synthesis decomposition
Answers: 1

Chemistry, 22.06.2019 05:20
Asolution contains 180 g of glucose (c6h12o6) and 162 g of water. what is the mole fraction of glucose?
Answers: 3

Chemistry, 22.06.2019 07:30
In a reaction (at equilibrium) that makes more moles of gas than it consumes, what is the effect of increasing the pressure?
Answers: 1

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 1
You know the right answer?
A5.018 gram sample of a certain hydrate of magnesium sulfate, mgso4•xh2o, is heated until all the wa...
Questions




Mathematics, 23.01.2021 06:30


Mathematics, 23.01.2021 06:30

Mathematics, 23.01.2021 06:30

Mathematics, 23.01.2021 06:30



French, 23.01.2021 06:30



English, 23.01.2021 06:30


Mathematics, 23.01.2021 06:30



Health, 23.01.2021 06:30

Advanced Placement (AP), 23.01.2021 06:30


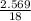

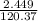
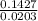 = 7.02 ~7
= 7.02 ~7

